DNase I-XT
Product information| Code | Name | Size | Quantity | Price | |
|---|---|---|---|---|---|
M0570S |
DNase I-XT |
1000 units ( 2000 units/ml ) | - | Unavailable in your region | |
M0570L |
DNase I-XT |
5000 units ( 2000 units/ml ) | - | Unavailable in your region |
DNase I-XT
Catalog #M0570
Product Introduction
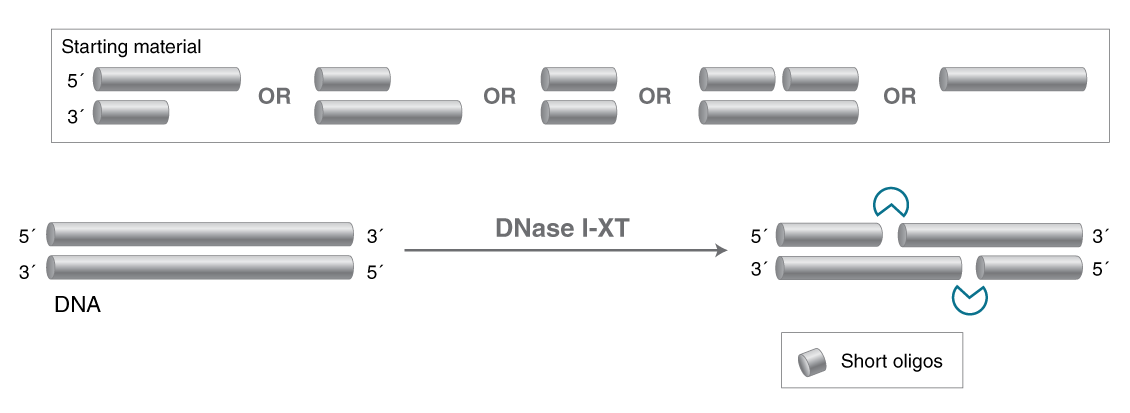
- Salt-tolerant DNA-specific endonuclease
- Degrades double-stranded and single-stranded DNA
- Generates short oligos with 5′-phosphate and 3′-OH
- RNase-free
- Need help finding the right exonuclease for your experiments? Try Exo Selector
DNase I-XT is ideal for:
- Degradation of DNA templates from in vitro transcription reactions
- Removal of contaminating genomic DNA from RNA samples
- Product Information
- Protocols, Manuals & Usage
- Tools & Resources
- FAQs & Troubleshooting
- Citations & Technical Literature
- Quality, Safety & Legal
Product Information
Description
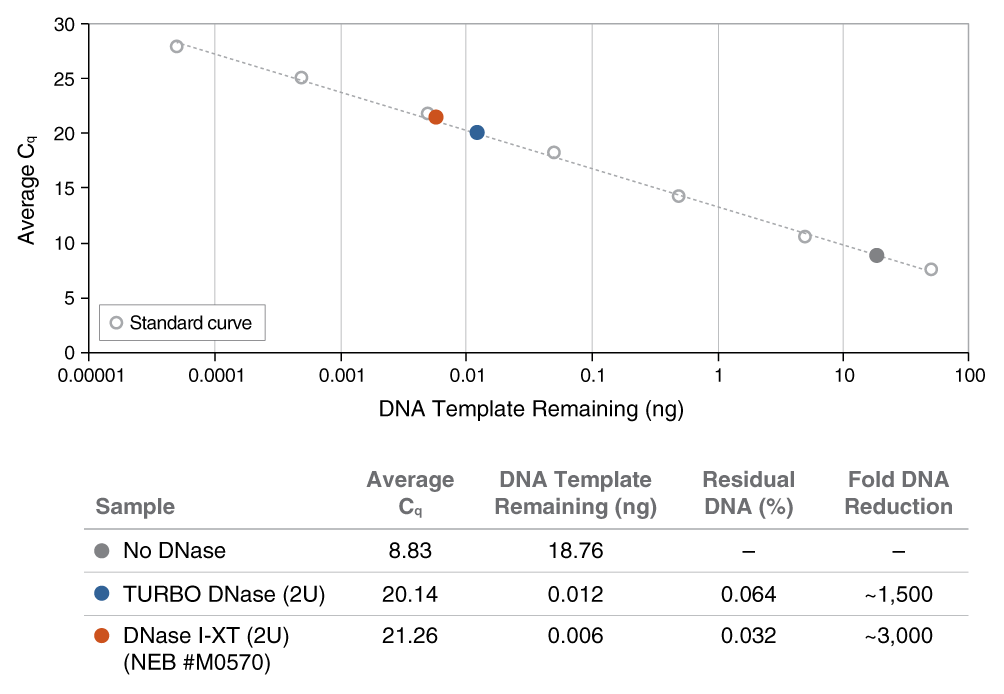
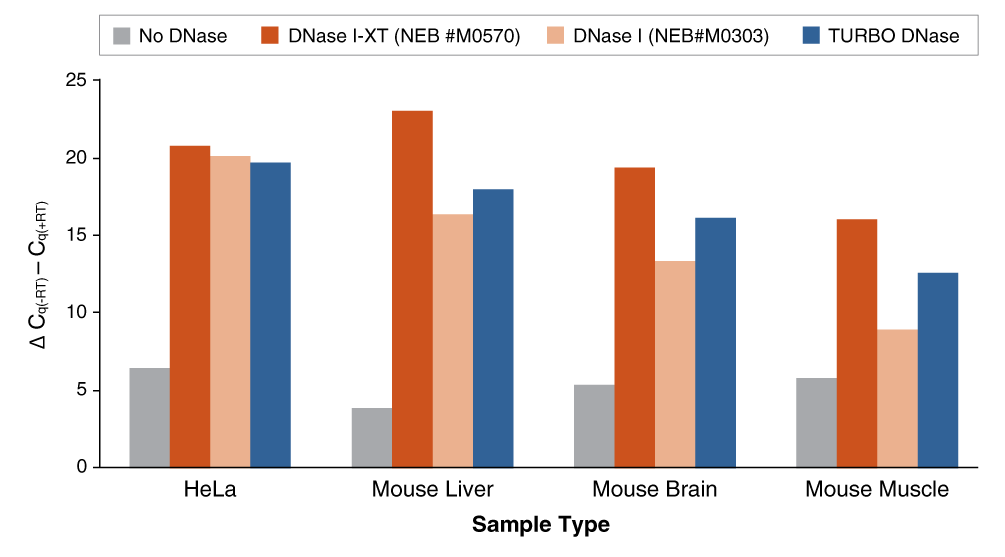
An engineered variant of DNase I, DNase I-XT is a salt-tolerant DNA endonuclease that nonspecifically cleaves DNA to release di-, tri- and oligonucleotide products with 5′-phosphorylated and 3′-hydroxylated ends (1,2). DNase I-XT acts on single- and double-stranded DNA, chromatin and the DNA strand of RNA:DNA hybrids. While DNase I (RNase-free) (NEB #M0303) is inhibited by salt concentrations >50 mM, DNase I-XT (NEB #M0570) exhibits optimal activity between 50-100 mM salt and retains 65% and ~40% activity in 200 and 300 mM salt, respectively. This increased salt tolerance makes DNase I-XT the preferred enzyme for DNA template removal from an in vitro transcription (IVT) reaction. Importantly, DNase I-XT is RNase-free, allowing for the complete removal of DNA from RNA preparations while maintaining RNA integrity.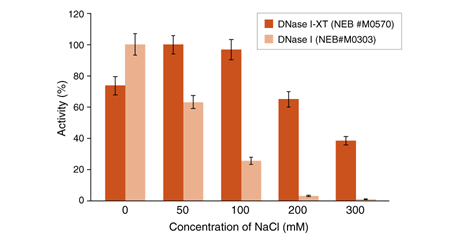
Product Source
A His-tagged engineered variant of DNase I expressed in Pichia pastoris.- This product is related to the following categories:
- Exonucleases and Non-specific Endonucleases,
- RNA Synthesis In vitro Transcription (IVT),
- Nucleotide Solutions for RNA,
Reagents Supplied
Reagents Supplied
The following reagents are supplied with this product:
| NEB # | Component Name | Component # | Stored at (°C) | Amount | Concentration | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
Properties & Usage
Unit Definition
One unit is defined as the amount of enzyme required to release 210 pmol of FAM from a 35mer FAM-BHQ1 labeled hairpin oligonucleotide in 1 minute at 30°C in a 50 µl reaction volume.Reaction Conditions
1X DNase I-XT Reaction Buffer
Incubate at 37°C
1X DNase I-XT Reaction Buffer
10 mM Tris-HCl
50 mM NaCl
10 mM MgCl2
0.5 mM CaCl2
0.005% Tween® 20
(pH 7.6 @ 25°C)
Storage Buffer
10 mM Tris-HCl
2 mM CaCl2
50% Glycerol
pH 7.6 @ 25°C
Heat Inactivation
NoFeatures
- Use in reactions containing higher amounts of salt, such as IVT and RNA preps
- Add directly to your IVT reaction, with no dilution required
- Efficiently removes DNA from IVT reactions and RNA preps
- RNase-free enzyme tolerates a wide range of salt conditions (up to 300 mM)
Related Products
Companion Products
- DNase I (RNase-free)
- HiScribe®; T7 Quick High Yield RNA Synthesis Kit
- Monarch® RNA Cleanup Kit (50 µg)
- HiScribe®; T7 Quick High Yield RNA Synthesis Kit
Materials Sold Separately
Product Notes
- DNase I-XT is supplied with an optimized reaction buffer for DNase I-XT. For optimal activity, we recommend using DNase I-XT (NEB #M0570) with DNase I-XT Reaction Buffer (NEB #B0570) and not with DNase I Reaction Buffer (NEB #B0303). Likewise, due to the salt in DNase I-XT Reaction Buffer being inhibitory to DNase I, we do not recommend use of DNase I-XT Reaction Buffer (NEB #B0570) with DNase I (NEB #M0303).
- We do not recommend using NEB's DNase I-XT as a substitute for Monarch DNase I in the RNA isolation workflow when using the Monarch Total RNA Miniprep Kit (NEB #T2010). Monarch DNase I is optimized for use in this workflow, and is available for purchase as a component of the Monarch Total RNA Enzyme Pack, (NEB #T2019).
References
- Kunitz, M. (1950). J. Gen. Physiol.. 33, 349-362.
- Vanecko, S. and laskowski, M. (1961). J. Biol. Chem.. 236, 3312-3316.
Protocols, Manuals & Usage
Protocols
Usage & Guidelines
Tools & Resources
Selection Charts
FAQs & Troubleshooting
FAQs
- What is the difference between DNase I (RNase-free) (NEB #M0303) and DNase I-XT (NEB #M0570)?
- Will DNase I-XT degrade more than 1 µg of DNA in an IVT reaction?
- Will DNase I-XT work in NEBuffer r1.1, NEBuffer r2.1, NEBuffer r3.1, or rCutSmart?
- What is the best way to remove DNase I-XT from my reaction?
- Will DNase I-XT work in rCutSmart buffer?
- Can I use the Monarch RNA Cleanup Kit (NEB #T2040) to clean up my DNase I-XT-treated RNA?
- Can I use NEB DNase I-XT with the Monarch Total RNA Miniprep Kit (NEB #T2010)?
Citations & Technical Literature
Citations
Quality, Safety & Legal
Quality Assurance Statement
Quality Control tests are performed on each new lot of NEB product to meet the specifications designated for it. Specifications and individual lot data from the tests that are performed for this particular product can be found and downloaded on the Product Specification Sheet, Certificate of Analysis, data card or product manual. Further information regarding NEB product quality can be found here.Specifications
The Specification sheet is a document that includes the storage temperature, shelf life and the specifications designated for the product. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]Certificate Of Analysis
The Certificate of Analysis (COA) is a signed document that includes the storage temperature, expiration date and quality controls for an individual lot. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]_[Lot Number]- M0570L_v2_10135978
- M0570S_v2_10135979
- M0570L_v2_10191288
- M0570S_v2_10191289
- M0570S_v2_10211604
- M0570S_v2_10222861
- M0570S_v2_10212823
- M0570L_v2_10226675
- M0570S_v2_10236903
- M0570L_v2_10245593
- M0570S_v2_10245592
- M0570L_v2_10250969
- M0570S_v2_10258343
- M0570L_v2_10258342
- M0570L_v2_10267519
- M0570L_v2_10275828
- M0570L_v2_10282189
- M0570S_v2_10282188
- M0570L_v2_10291483
- M0570L_v2_10300211
- M0570S_v2_10298988
Safety DataSheets
The following is a list of Safety Data Sheet (SDS) that apply to this product to help you use it safely.DNase I-XT
DNase I-XT Reaction Buffer
Legal and Disclaimers
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email [email protected].This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
New England Biolabs (NEB) is committed to practicing ethical science – we believe it is our job as researchers to ask the important questions that when answered will help preserve our quality of life and the world that we live in. However, this research should always be done in safe and ethical manner. Learn more.
The supporting documents available for this product can be downloaded below.




