HiScribe® T7 Quick High Yield RNA Synthesis Kit
Product information| Code | Name | Size | Quantity | Price | |
|---|---|---|---|---|---|
E2050S |
HiScribe T7 Quick High Yield RNA Synthesis Kit |
50 rxns | - | Unavailable in your region | |
E2050L |
HiScribe T7 Quick High Yield RNA Synthesis Kit |
250 rxns | - | Unavailable in your region |
HiScribe® T7 Quick High Yield RNA Synthesis Kit
Now includes separate tube of DTT
Product Introduction
- Up to 180 μg of RNA generated per reaction, from 1 μg of control template
- Master mix format reduces pipetting steps while still enabling partial substitution of NTPs for labeling and incorporation of modified bases including N1-Methyl-Pseudouridine-5’-Triphosphate (NEB #N0431), 5-Methyl-Cytidine-5’-Triphosphate (NEB #N0432), Pseudouridine-5’-Triphosphate (NEB #N0433) and 5-Methoxy-Uridine-5’-Triphosphate (NEB #N0434)
- Template removal and RNA purification reagents included
- Linearized control template included for verification of RNA synthesis
- Getting ready to scale up RNA synthesis? Download our new technical note “Scaling of High-Yield In vitro Transcription Reactions for Linear Increase of RNA Production” for a generalized set of recommendations for synthesizing high yields of RNA.
| Catalog # | Size | Concentration |
|---|---|---|
| E2050S | 50 reactions |
Featured Videos
View Video Library- Product Information
- Protocols, Manuals & Usage
- FAQs & Troubleshooting
- Citations & Technical Literature
- Quality, Safety & Legal
- Other Products You May Be Interested In
Product Information
Description
The HiScribe T7 Quick High Yield RNA Synthesis Kit is designed for quick set-up and production of large amounts of RNA in vitro. The reaction can be set up conveniently by combining the NTP buffer mix, T7 RNA Polymerase mix and a suitable DNA template. The kit also allows for capped RNA or dye-labeled RNA synthesis by incorporation of cap analog (ARCA, NEB #S1411) or dye-modified nucleotides. RNA synthesized with the kit can be used for RNA structure and function studies, ribozyme biochemistry, as probes for RNase protection assays and hybridization based blots, anti-sense RNA and RNAi experiments, microarray analysis and microinjection, as well as in vitro translation and RNA vaccines.To synthesize high specific activity radioactive RNA probes or RNA with 100% substitution of one or more modified nucleotides we recommend using the T7 High Yield RNA Synthesis Kit (NEB #E2040), in which the four nucleotides are supplied separately.
DNA Template Preparation:
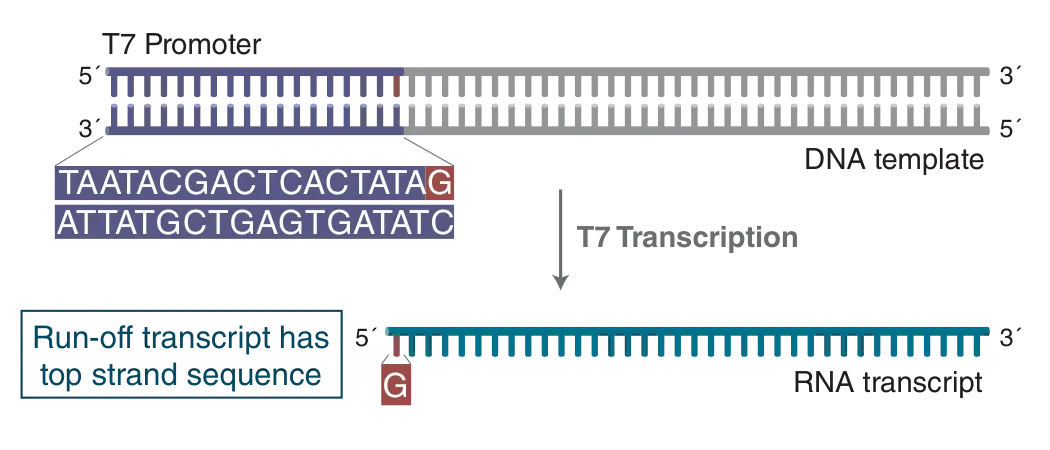
Linearized plasmid DNA, PCR products or synthetic DNA oligonucleotides can be used as templates for in vitro transcription with the HiScribe T7 Quick High Yield RNA Synthesis Kit, provided that they contain a double-stranded T7 promoter region upstream of the sequence to be transcribed. Figure 1 illustrates the minimal T7 promoter sequence, as well as a run-off transcript after T7 transcription.
- This product is related to the following categories:
- sgRNA Synthesis,
- RNA Synthesis In vitro Transcription (IVT),
- Nucleotide Solutions for RNA,
Kit Components
Kit Components
The following reagents are supplied with this product:
| NEB # | Component Name | Component # | Stored at (°C) | Amount | Concentration | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
Properties & Usage
Materials Required but not Supplied
- Nuclease-free Water (NEB #B1500)
Related Products
Companion Products
- RNA Loading Dye, (2X)
- RNase Inhibitor, Human Placenta
- RNase Inhibitor, Murine
- DNase I (RNase-free)
- Q5® Hot Start High-Fidelity DNA Polymerase
- ssRNA Ladder
- Low Range ssRNA Ladder
- 3´-0-Me-m7G(5')ppp(5')G RNA Cap Structure Analog
- m7G(5')ppp(5')A RNA Cap Structure Analog
- G(5')ppp(5')A RNA Cap Structure Analog
- G(5')ppp(5')G RNA Cap Structure Analog
- m7G(5')ppp(5')G RNA Cap Structure Analog
- Vaccinia Capping System
- mRNA Cap 2 O Methyltransferase
- E. coli Poly(A) Polymerase
- Ribonucleotide Solution Mix
- Ribonucleotide Solution Set
- Monarch® RNA Cleanup Kit (50 µg)
- Uracil Glycosylase Inhibitor (UGI)
- N1-Methyl-Pseudouridine-5´-Triphosphate (N1-Methyl-Pseudo-UTP)
- 5-Methyl-Cytidine-5´-Triphosphate (5-Methyl-CTP)
- Pseudouridine-5´-Triphosphate (Pseudo-UTP)
- 5-Methoxy-Uridine-5´-Triphosphate (5-Methoxy-UTP)
Protocols, Manuals & Usage
Protocols
Manuals
The Product Manual includes details for how to use the product, as well as details of its formulation and quality controls.
Application Notes
FAQs & Troubleshooting
FAQs
Troubleshooting
- Control Reaction
The FLuc control template DNA is a linearized plasmid containing the firefly luciferase gene under the transcriptional control of the T7 promoter. The size of the runoff transcript is 1.8 kb. The control reaction should yield ≥ 150 μg RNA transcript in 2 hours.
If the control reaction is not working, there may be technical problems during reaction set up. Repeat the reaction by following the protocol carefully; take all precautions to avoid RNase contamination. Contact NEB for technical assistance.
The control plasmid sequence can be found within the DNA Sequences and Maps Tool under the name “FLuc Control Plasmid”. The FLuc control template is generated by linearizing the plasmid with StuI.
- Low Yield of Full-length RNA
If the transcription reaction with your template generates full-length RNA, but the yield is significantly lower than expected, it is possible that contaminants in the DNA template are inhibiting the RNA polymerase, or the DNA concentration may be incorrect. Alternatively, additional purification of DNA template may be required. Phenol:chloroform extraction is recommended (see template DNA preparation section).
- Low Yield of Short Transcript
High yields of short transcripts (< 0.3 kb) are achieved by extending incubation time and increasing the amount of template. Incubation of reactions up to 16 hours (overnight) or using up to 2 μg of template will help to achieve maximum yield.
- RNA Transcript Smearing on Denaturing Gel
If the RNA appears degraded (e.g., smeared) on denaturing agarose or polyacrylamide gel, the DNA template is likely contaminated with RNase. DNA templates contaminated with RNase can affect the length and yield of RNA synthesized (a smear below the expected transcript length). If the plasmid DNA template is contaminated with RNase, perform phenol:chloroform extraction, then ethanol precipitate and dissolve the DNA in nuclease-free water (see template DNA preparation section).
- RNA Transcript of Larger Size than Expected
If the RNA transcript appears larger than expected on a denaturing gel, template plasmid DNA may be incompletely digested. Even small amounts of undigested circular DNA can produce large amounts of long transcripts. Check template for complete digestion. If undigested plasmid is confirmed, repeat restriction enzyme digestion.
Larger size bands may also be observed when the RNA transcript is not completely denatured due to the presence of strong secondary structures.
- RNA Transcript of Smaller Size than Expected
If denaturing gel analysis shows the presence of smaller bands than the expected size, it is most likely due to premature termination by the polymerase. Some sequences with resemblance to T7 RNA Polymerase termination signals will cause premature termination. Incubating the transcription reaction at lower temperatures, for example at 30°C, may increase the proportion of full-length transcript, however the yield will be decreased. For GC rich templates, or templates with secondary structures, incubation at 42°C may improve yield of full-length transcript.
Citations & Technical Literature
Citations
Additional Citations
Quality, Safety & Legal
Quality Assurance Statement
Quality Control tests are performed on each new lot of NEB product to meet the specifications designated for it. Specifications and individual lot data from the tests that are performed for this particular product can be found and downloaded on the Product Specification Sheet, Certificate of Analysis, data card or product manual. Further information regarding NEB product quality can be found here.Specifications
The Specification sheet is a document that includes the storage temperature, shelf life and the specifications designated for the product. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]Certificate Of Analysis
The Certificate of Analysis (COA) is a signed document that includes the storage temperature, expiration date and quality controls for an individual lot. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]_[Lot Number]- E2050S_v1_0161803
- E2050S_v1_10014822
- E2050S_v1_10013322
- E2050S_v1_10025870
- E2050S_v1_10032541
- E2050S_v1_10032887
- E2050S_v1_10034410
- E2050S_v1_10040045
- E2050S_v1_10038950
- E2050S_v1_10046640
- E2050S_v1_10048470
- E2050S_v1_10051805
- E2050S_v1_10056893
- E2050S_v1_10059533
- E2050S_v1_10064629
- E2050S_v1_10066796
- E2050S_v1_10067931
- E2050S_v1_10070259
- E2050S_v1_10073630
- E2050S_v1_10079440
- E2050S_v1_10082353
- E2050S_v1_10087154
- E2050S_v1_10096709
- E2050S_v1_10099443
- E2050S_v1_10107625
- E2050S_v1_10109520
- E2050S_v1_10114674
- E2050S_v1_10117542
- E2050S_v1_10125176
- E2050S_v1_10131565
- E2050S_v1_10138224
- E2050S_v1_10143401
- E2050S_v1_10148494
- E2050S_v1_10152993
- E2050S_v1_10157844
- E2050S_v1_10164820
- E2050S_v1_10169126
- E2050S_v1_10174083
- E2050S_v1_10176424
- E2050S_v1_10179750
- E2050S_v1_10184687
- E2050S_v1_10186994
- E2050S_v1_10188127
- E2050S_v1_10200287
- E2050S_v1_10202942
- E2050S_v2_10203846
- E2050S_v2_10215190
- E2050S_v2_10225736
- E2050S_v2_10228526
- E2050S_v3_10230843
- E2050S_v3_10238455
- E2050L_v1_10241176
- E2050S_v3_10240130
- E2050S_v3_10249324
- E2050L_v1_10255994
- E2050S_v3_10256953
- E2050L_v1_10265426
- E2050L_v1_10270360
- E2050L_v1_10273067
- E2050S_v3_10276293
- E2050L_v1_10261683
- E2050S_v3_10277000
- E2050L_v1_10286840
- E2050S_v3_10285870
- E2050L_v1_10290201
- E2050S_v3_10296491
- E2050L_v1_10292870
- E2050S_v3_10302246
- E2050L_v1_10307603
Safety DataSheets
The following is a list of Safety Data Sheet (SDS) that apply to this product to help you use it safely.FLuc Control Template
DNase I (RNase-free)
T7 RNA Polymerase Mix
LiCl Solution
NTP Buffer Mix
Dithiothreitol (DTT)
Legal and Disclaimers
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email [email protected].This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
New England Biolabs (NEB) is committed to practicing ethical science – we believe it is our job as researchers to ask the important questions that when answered will help preserve our quality of life and the world that we live in. However, this research should always be done in safe and ethical manner. Learn more.
The supporting documents available for this product can be downloaded below.
Protocol
Capped RNA Synthesis (E2050)
Protocol
Standard RNA Synthesis (E2050)
Protocol
Standard RNA Synthesis (E2050)
Protocol
Standard RNA Synthesis (E2050)
Protocol
Standard RNA Synthesis (E2050)
Protocol
Capped RNA Synthesis (E2050)
Protocol
Purification of IVT RNA
Protocol
Evaluation of IVT Reaction Products
Protocol
Capped RNA Synthesis (E2050)
Protocol
Capped RNA Synthesis (E2050)