SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit
Product information| Code | Name | Size | Quantity | Price | |
|---|---|---|---|---|---|
E2019S |
SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit |
96 rxns | - | Unavailable in your region |
SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit
For Research Use Only. Not for use in diagnostic procedures.
Catalog #E2019
Product Introduction
The SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit utilizes isothermal amplification for use in the analysis of SARS-CoV-2, the novel coronavirus that causes COVID-19.
- What is the impact of the Omicron variant on this assay? See the new FAQ and view the Primer Monitor Tool (more info here) for up-to-date information on variants mapped against common primer sets.
- Colorimetric LAMP enables simple, visual detection (pink-to-yellow) of amplification of SARS-CoV-2 nucleic acid
- Set up reactions quickly and easily using a simple heat source and unique WarmStart® technology
- Reduce risk of carryover contamination with thermolabile UDG and dUTP included in the master mix
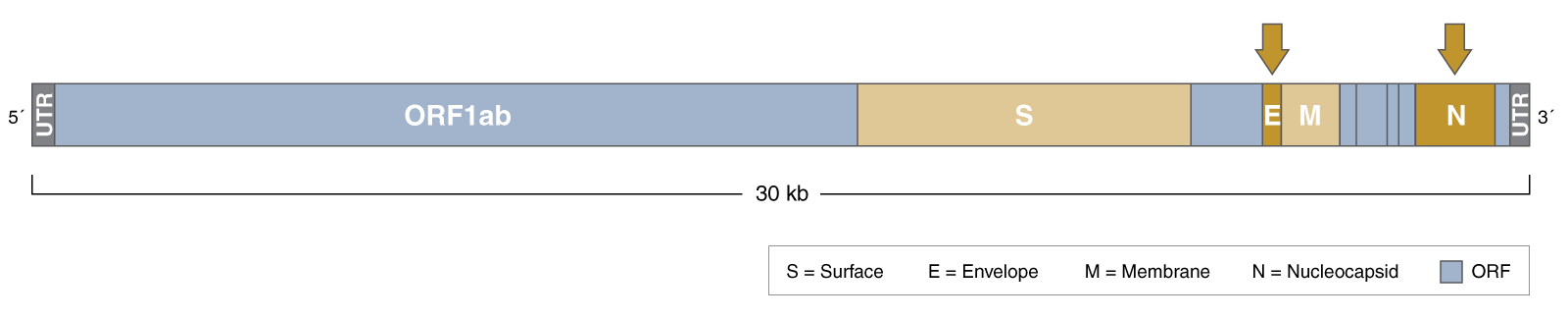
- Assay targets N and E regions of the SARS-CoV-2 genome for optimized sensitivity and specificity
- Bring confidence to your results using the provided controls
- Components sold separately
- Learn more about LAMP and other isothermal amplification methods
- Need assistance designing LAMP primers? Use the NEB LAMP Primer Design Tool
- Read how NEB developed and implemented a SARS-CoV-2 RT-LAMP workflow for workplace surveillance: 1) NEB Expressions article 2) PLOS ONE publication
- A publication by members of the global LAMP (gLAMP) Consortium provides a comprehensive review of LAMP and its role in the COVID-19 pandemic
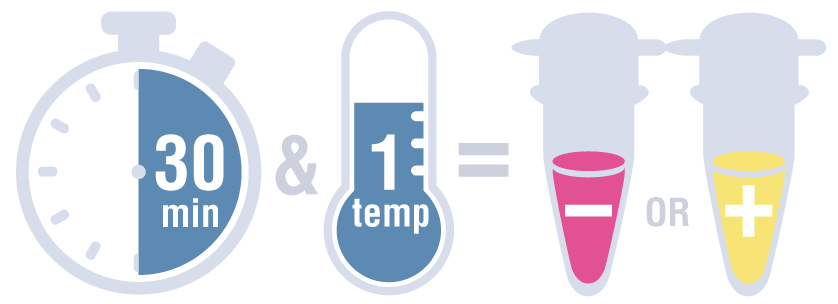
- Product Information
- Protocols, Manuals & Usage
- Tools & Resources
- FAQs & Troubleshooting
- Citations & Technical Literature
- Quality, Safety & Legal
- Other Products You May Be Interested In
Product Information
Description
The SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit utilizes Loop-Mediated Isothermal Amplification (LAMP) to detect SARS-CoV-2 nucleic acid. The kit is available for research use only and includes WarmStart Colorimetric LAMP 2X Master Mix with UDG and a primer mix targeting the N and E regions of the viral genome. Controls are provided to verify assay performance, and include an internal control primer set and a positive control template. Guanidine hydrochloride has been found to increase the speed and sensitivity of the RT-LAMP reaction and is also included.
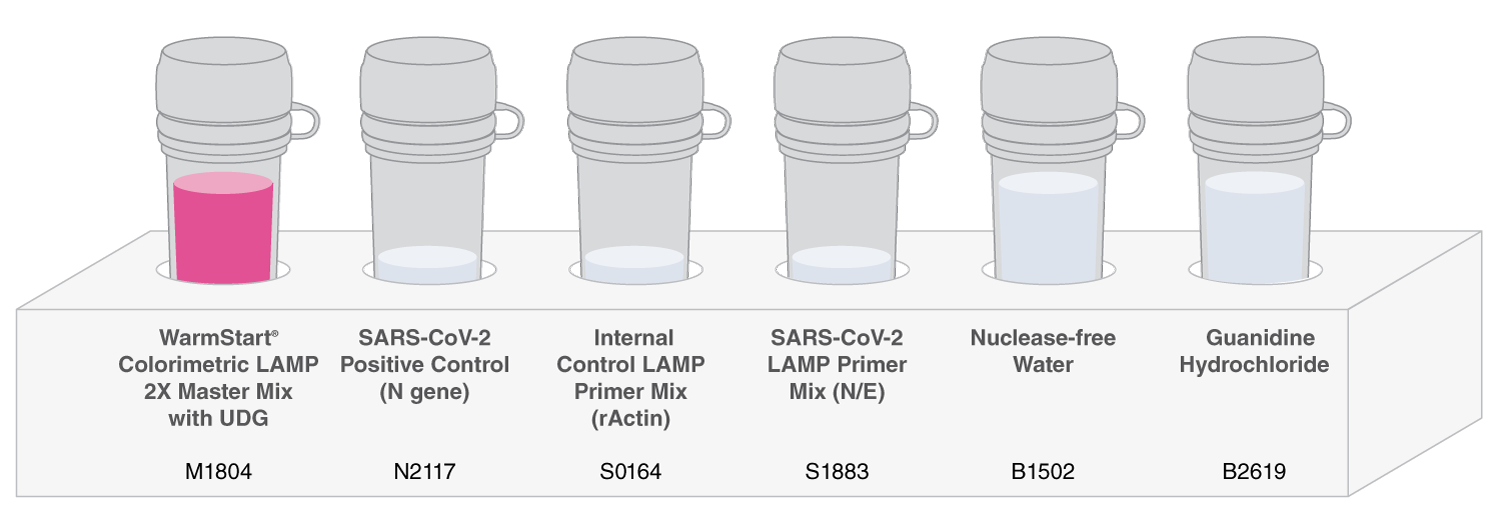
WarmStart Colorimetric LAMP 2X Master Mix with UDG is an optimized formulation of Bst 2.0 WarmStart DNA Polymerase and WarmStart RTx in a special low-buffer reaction solution containing a visible pH indicator for rapid and easy detection of LAMP and RT-LAMP reactions. The inclusion of thermolabile UDG and dUTP in the master mix reduces the possibility of carryover contamination between reactions.
.
This system provides a fast, clear visual detection of amplification based on the production of protons and the subsequent drop in pH that occurs from the extensive DNA polymerase activity in a LAMP reaction. The decrease in pH produces a change in solution color from pink to yellow. The total protocol time is 30 minutes and only requires a simple heating device that can reach 65°C.
.
A positive detection of the SARS-CoV-2 RNA sequence would be indicated by a yellow color (amplification occurred, protons released, pH-dependent color change from pink to yellow), while a negative result would be indicated by a pink color (no amplification occurred, no protons released, no color change).
.
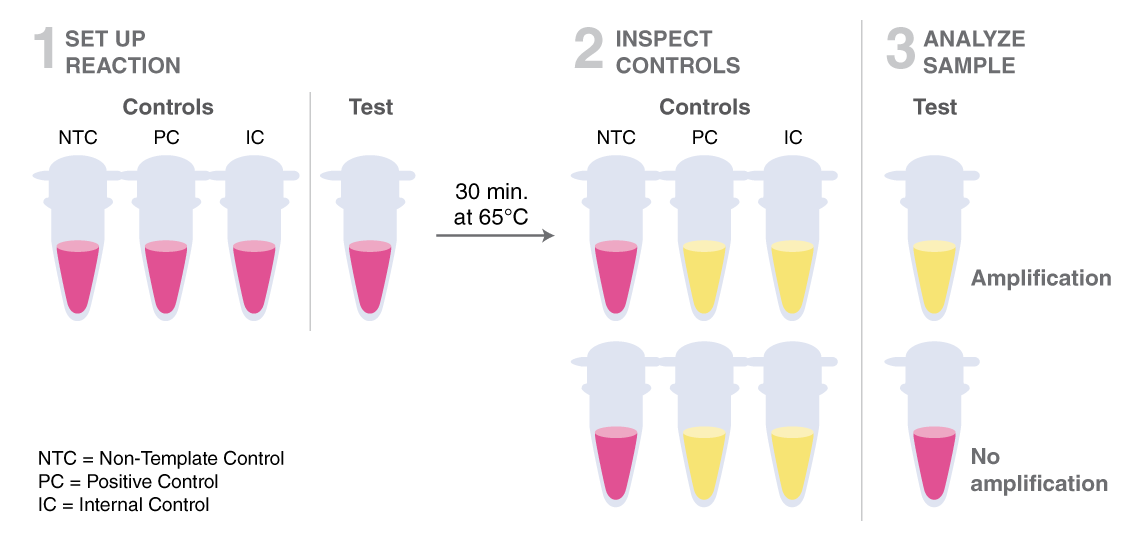
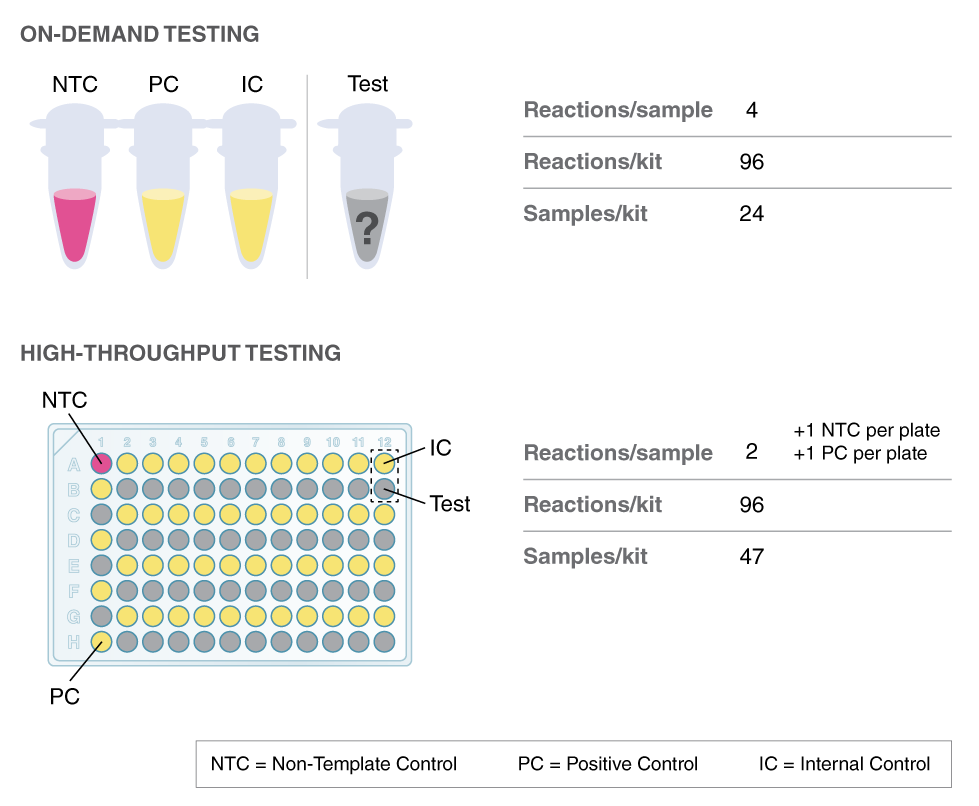
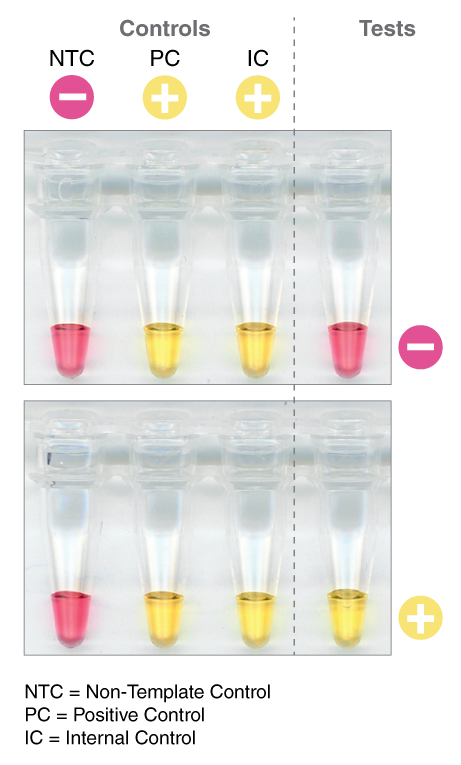
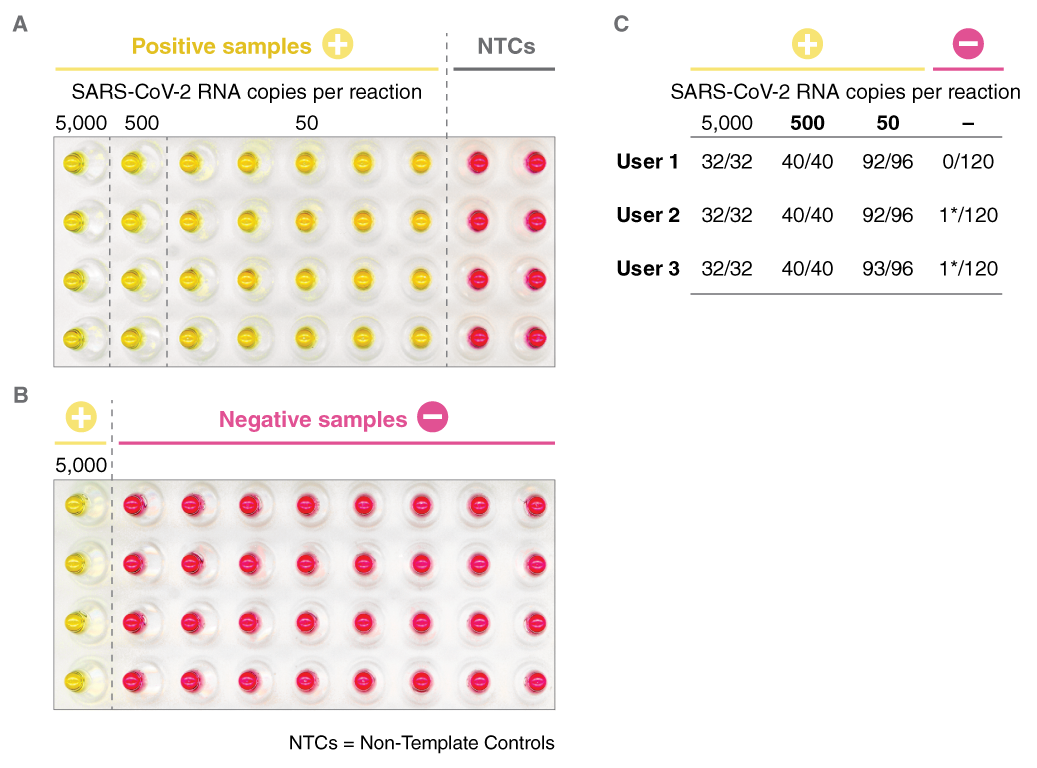
LAMP has been used in novel workflows by scientists around the world to further COVID-19 research. To access application notes, selected preprints, and to learn more about NEB’s COVID-19 Researcher Spotlight series, click here.
- This product is related to the following categories:
- Isothermal Amplification & Strand Displacement,
- PCR, qPCR & Amplification Technologies,
- This product can be used in the following applications:
- Loop-Mediated Isothermal Amplification,
- Isothermal Amplification,
- DNA Amplification, PCR & qPCR
Kit Components
Kit Components
The following reagents are supplied with this product:
| NEB # | Component Name | Component # | Stored at (°C) | Amount | Concentration | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Properties & Usage
Materials Required but not Supplied
Disposable powder-free gloves and any additional PPE required
P2/P10, P200, and P1000 aerosol barrier tips
Sterile, nuclease-free 1.5 ml microcentrifuge tubes
Sterile, nuclease-free 2.0 ml, 5.0 ml, or 15 ml tubes
0.2 ml PCR reaction tube strips with separated tubes and lids (e.g., VWR 20170-004) or attached caps (e.g., VWR 20170-010) or 96-well PCR reaction plates with 8-cap strips
Racks for 1.5 ml microcentrifuge tubes and 96-well 0.2 ml PCR reaction tubes
Cooler rack for 1.5 microcentrifuge tubes and 96-well 0.2 ml PCR reaction tubes
Acceptable surface decontaminants, for example: 10% bleach (1:10 dilution of commercial 5.25-6.0% sodium hypochlorite)
Laboratory marking pen
Appropriate disposal containers
White paper or light background for optimal visualization of colorimetric reaction (e.g., typical printer paper)
Micropipettes (2 or 10 μl, 200 μl and 1,000 μl), Multichannel Micropipettes (5-50 μl)
-20°C Freezer (frost-free or nonfrost), 4°C Refrigerator
Thermocycler, heat block or device that can be set to 65°C
PCR Work Station [UV lamp; Laminar flow (Class 100 HEPA filtered)], Vortex Mixer, Tabletop Microcentrifuge
Features
- 30-minute isothermal protocol – no sophisticated lab equipment required
- Colorimetric, visual detection – ease of use, simple-to-read results
- UDG and dUTP included in master mix for carryover prevention – reduced risk of contamination
- Optimized dual primer mix – enhanced detection and sensitivity
- Dual WarmStart formulation – room temperature setup
- Necessary controls included – verify assay performance for increased confidence in results
Related Products
Companion Products
Product Notes
-
Carryover Contamination Prevention
LAMP is a sensitive method that generates large quantities of DNA, and contamination in new LAMP assays with products from previous amplification reactions can cause a variety of issues, such as false positive results and a decrease in sensitivity. The best way to prevent this “carryover” contamination is to practice good laboratory procedures and avoid opening the reaction vessel post amplification. However, to accommodate situations where additional anti-contamination measures are desired, WarmStart Colorimetric LAMP Master Mix with UDG contains a mixture of dUTP/dTTP that results in the incorporation of dU into the DNA product during amplification. Antarctic Thermolabile Uracil DNA Glycosylase (UDG) present in the colorimetric mix will eliminate previously-amplified uracil-containing products by excising the uracil base to produce a non-amplifiable DNA product. The use of a thermolabile UDG is important, as quick and complete inactivation of the UDG is required to prevent destruction of newly synthesized LAMP products. To maximize elimination of contaminating products, set up colorimetric LAMP experiments at room temperature or include a 10 minute incubation step at 25°C before isothermal incubation.
-
Reaction Setup and Isothermal Incubation
Due to the dual WarmStart feature of the Colorimetric LAMP Master Mix with UDG, reactions may be set up at room temperature. Room temperature set up will also allow UDG to destroy previously generated dU-containing LAMP products.
- We recommend a final reaction volume of 25 μl.
- For maximum sensitivity, go directly from room temperature to a preheated block set at 65°C. Reactions should not be allowed to sit on a block as it warms to 65°C.
Protocols, Manuals & Usage
Protocols
Manuals
Application Notes
Tools & Resources
Web Tools
FAQs & Troubleshooting
FAQs
- How does the new Omicron variant impact the effectiveness of the SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit?
- Can the Colorimetric LAMP 2X Master Mix be used in instruments that utilize fluorescent detection of DNA amplification or real time turbidity measurement?
- How stable are the WarmStart Colorimetric LAMP 2X Master Mixes?
- The WarmStart LAMP Master Mix has some precipitation in the tube after thawing, is this normal?
- What if my colorimetric LAMP mix is orange instead of pink prior to amplification?
- Does the WarmStart® Colorimetric LAMP Master Mix with UDG enable carryover prevention/contamination reduction?
- Does pre-heating the thermal block impact LAMP amplification reactions?
- What regions of the SARS-CoV-2 virus do the LAMP Primers target? How were these primers chosen?
- In the SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit, what is the target of the Internal Control Primer Mix (rActin)?
- Should I use Guanidine HCl (B2619A) in colorimetric LAMP reactions?
- How are colorimetric LAMP results interpreted in the SARS-CoV-2 Kit?
- After amplification my samples turned orange rather than yellow. Is this acceptable? How do I interpret the results?
- Can I incubate my colorimetric LAMP Sars-CoV-2 samples longer than 30 minutes at 65°C? How long should I incubate the reaction before checking the results?
- My NTC sample(s) turned orange/yellow following incubation at 65°C. What happened?
- My colorimetric LAMP SARS-CoV-2 Positive Control failed to turn yellow following incubation at 65°C. What happened?
- What is the SARS-CoV-2 Positive Control included in the kit?
- How much sample can be added to the colorimetric LAMP reaction as input?
- What types of samples/materials are compatible with the SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit?
- My SARS-CoV-2 sample or Internal Control reaction turned orange/yellow upon sample addition. Does that mean that the sample contains nucleic acid?
- What instruments are compatible with the SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit?
- Can I monitor the color change of the colorimetric LAMP reactions using a spectrophotometer?
- Can the WarmStart Colorimetric LAMP 2X Master Mixes be used in instruments that utilize fluorescence detection?
Troubleshooting
Citations & Technical Literature
Citations
Feature Articles
Quality, Safety & Legal
Quality Assurance Statement
Quality Control tests are performed on each new lot of NEB product to meet the specifications designated for it. Specifications and individual lot data from the tests that are performed for this particular product can be found and downloaded on the Product Specification Sheet, Certificate of Analysis, data card or product manual. Further information regarding NEB product quality can be found here.Specifications
The Specification sheet is a document that includes the storage temperature, shelf life and the specifications designated for the product. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]Certificate Of Analysis
The Certificate of Analysis (COA) is a signed document that includes the storage temperature, expiration date and quality controls for an individual lot. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]_[Lot Number]- E2019S_v1_10084875
- E2019S_v1_10086106
- E2019S_v1_10088109
- E2019S_v1_10091674
- E2019S_v2_10095828
- E2019S_v2_10098080
- E2019S_v2_10099767
- E2019S_v2_10104430
- E2019S_v2_10112519
- E2019S_v2_10120897
- E2019S_v2_10139608
- E2019S_v2_10153693
- E2019S_v2_10165688
- E2019S_v2_10170676
- E2019S_v2_10176903
- E2019S_v2_10186848
- E2019S_v2_10190486
- E2019S_v2_10237753
- E2019S_v2_10256784
Safety DataSheets
The following is a list of Safety Data Sheet (SDS) that apply to this product to help you use it safely.WarmStart® Colorimetric LAMP 2X Master Mix with UDG
SARS-CoV-2 Positive Control (N gene)
Control LAMP Primer Mix (rActin)
SARS-CoV-2 LAMP Primer Mix (N/E)
Nuclease-free Water
Guanidine Hydrochloride
Legal and Disclaimers
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email [email protected].This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
New England Biolabs (NEB) is committed to practicing ethical science – we believe it is our job as researchers to ask the important questions that when answered will help preserve our quality of life and the world that we live in. However, this research should always be done in safe and ethical manner. Learn more.
Licenses
This product is not available for sale in China (including Hong Kong and Macau), Japan and Taiwan.
LAMP is a patented technology belonging to Eiken Chemical Co., Ltd and any other use other than research may require a license from Eiken Chemical Co., Ltd. The purchase of this product conveys to the purchaser the limited, non-transferable right, under intellectual property owned or controlled by New England Biolabs, Inc., to use the product to perform loop-mediated amplification (“LAMP”) and reverse transcription loop-mediated amplification (“RT-LAMP”) for investigational research and in vitro diagnostic tests for the detection of severe acute respiratory syndrome coronavirus, SARS-CoV-2.
Nucleic acid-based aptamers for use with thermophilic DNA polymerases and reverse transcriptase are licensed exclusively by New England Biolabs, Inc. from SomaLogic, Inc. New England Biolabs, Inc. gives the purchaser a non-exclusive license to the SARS-CoV-2 Rapid Colorimetric Assay Kit for research and in vitro diagnostic tests for the detection of severe acute respiratory syndrome coronavirus, SARS-CoV-2.
This product is not approved by the US Food and Drug Administration, or any foreign equivalent, for diagnostic use. It is the purchaser’s responsibility to ensure it has appropriate authorization for its particular use. For additional information or to inquire about commercial use, please contact [email protected].
The supporting documents available for this product can be downloaded below.