NEBNext® rRNA Depletion Kit (Bacteria) with RNA Sample Purification Beads
Product information| Code | Name | Size | Quantity | Price | |
|---|---|---|---|---|---|
E7860S |
NEBNext rRNA Depletion Kit (Bacteria) with RNA Sample Beads |
6 rxns | - | Unavailable in your region | |
E7860L |
NEBNext rRNA Depletion Kit (Bacteria) with RNA Sample Beads |
24 rxns | - | Unavailable in your region | |
E7860X |
NEBNext rRNA Depletion Kit (Bacteria) with RNA Sample Beads |
96 rxns | - | Unavailable in your region |
NEBNext® rRNA Depletion Kit (Bacteria) with RNA Sample Purification Beads
Product Introduction
Ribosomal RNA (rRNA) is highly abundant in bacterial samples, and its removal is desirable in order to reveal the biological significance of less abundant transcripts. Specific enrichment of bacterial mRNAs is challenging due to their lack of poly(A) tails, and so the converse - efficient and specific removal of bacterial rRNA – is necessary.
The NEBNext® rRNA Depletion Kit (Bacteria) employs the NEBNext RNase H-based RNA depletion workflow to target removal of rRNA from gram-positive and gram-negative organisms. The method is effective with intact and degraded RNA, from monocultures or samples with mixed bacterial species (e.g., metatranscriptomic).
For added convenience, this kit contains NEBNext RNA Sample Purification Beads (Agencourt® RNAClean® XP beads from Beckman Coulter).
- Efficient, specific depletion of bacterial rRNA (5S, 16S, 23S)
- Compatible with both gram-positive and gram-negative organisms
- Effective with monocultures and mix of bacterial species (e.g., metatranscriptome)
- Compatible with a broad range of input amounts: 10 ng - 1 µg
- Suitable for low-quality or high-quality RNA
- Fast workflow: 2 hours, with less than 10 minutes hands-on time
The kit is also available without RNAClean® XP beads.
| Catalog # | Size | Concentration |
|---|---|---|
| E7860S | 6 reactions | |
| E7860L | 24 reactions | |
| E7860X | 96 reactions |
- Product Information
- Protocols, Manuals & Usage
- Tools & Resources
- FAQs & Troubleshooting
- Citations & Technical Literature
- Quality, Safety & Legal
- Other Products You May Be Interested In
Product Information
Description
The great majority of RNA in bacteria is ribosomal RNA (rRNA). This highly abundant RNA can conceal the biological significance of less abundant transcripts, and so its efficient and specific removal is desirable.
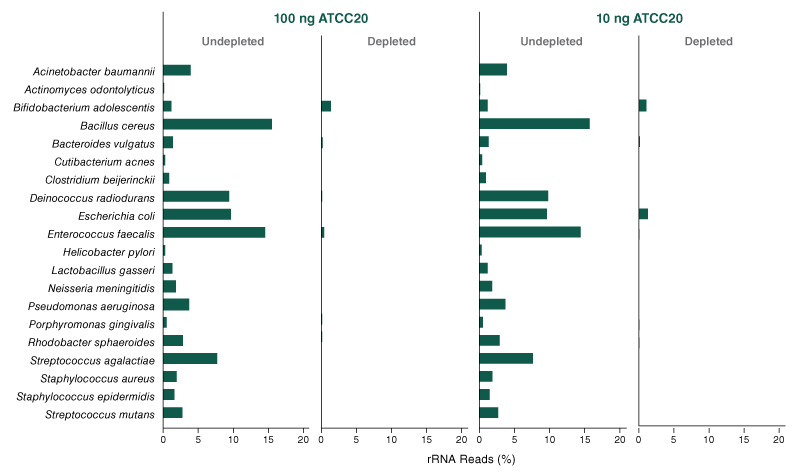
The NEBNext® rRNA Depletion Kit (Bacteria) employs the NEBNext RNase H-based RNA depletion workflow to deplete rRNA (5S, 16S, and 23S) from gram-positive and gram-negative organisms. View species tested to date.
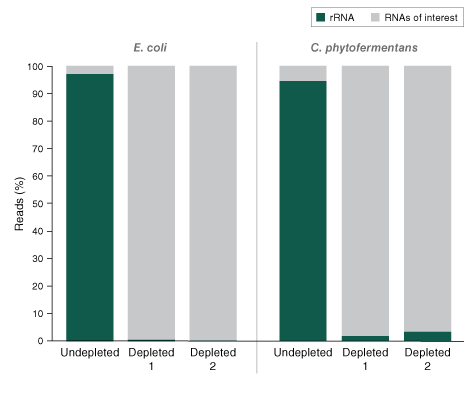
The kit is effective with both intact and degraded RNA preparations, from monocultures or samples with mixed bacterial species (e.g., metatranscriptomic).
The kit is also available without RNAClean® XP beads.
- Efficient, specific depletion of bacterial rRNA (5S, 16S, 23S)
- Compatible with both gram-positive and gram-negative organisms.
- Effective with monocultures and mix of bacterial species (e.g., metatranscriptome)
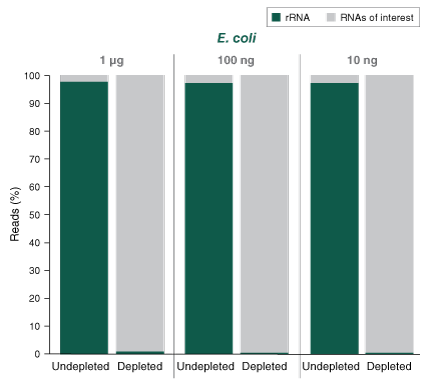
- Compatible with a broad range of input amounts: 10 ng - 1 µg
- Suitable for low-quality or high-quality RNA
- Fast workflow: 2 hours, with less than 10 minutes hands-on time
- Includes NEBNext RNA Sample Purification Beads (Agencourt® RNAClean® XP)
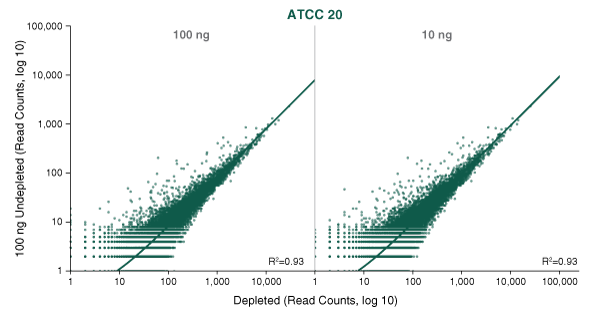
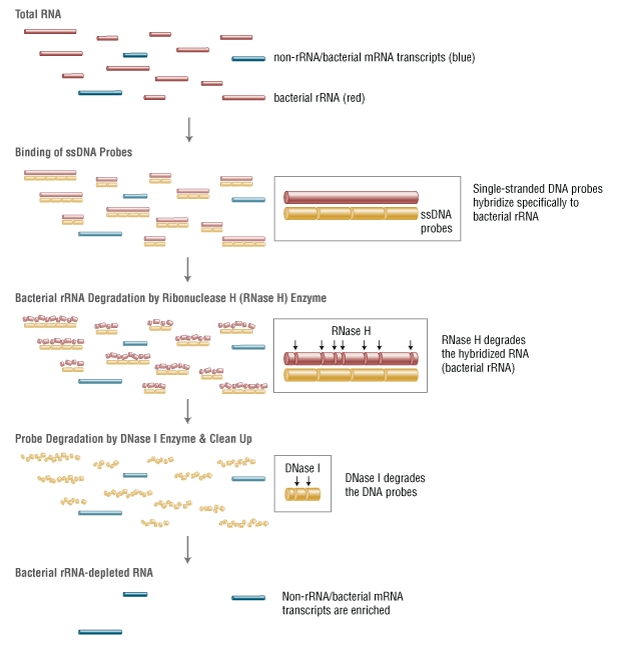
- This product is related to the following categories:
- RNA Depletion & mRNA Enrichment,
- RNA Library Prep for Illumina,
- Epitranscriptome Analysis,
- Next Generation Sequencing Library Preparation,
Kit Components
Kit Components
The following reagents are supplied with this product:
| NEB # | Component Name | Component # | Stored at (°C) | Amount | Concentration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Properties & Usage
Materials Required but not Supplied
- Magnetic rack (NEB #S1515) or magnetic plate (Alpaqua. cat. #A001322) or equivalent
- 80% Ethanol (freshly prepared)
- Thermocycler
- Any thin wall 200 μl PCR tubes and 1.5 ml tubes
- Bioanalyzer, Tapestation. (Agilent Technologies, Inc.) or similar instrument and consumables
Protocols, Manuals & Usage
Manuals
Usage & Guidelines
Tools & Resources
Web Tools
FAQs & Troubleshooting
FAQs
- Which bacterial rRNA subunits are depleted with the NEBNext rRNA Depletion Kit Bacteria
- Can the NEBNext® rRNA Depletion Kit (Bacteria) be used on metatranscriptomic samples?
- How can I remove rRNA from a mix of mammalian and bacterial samples
- What is the percentage of bacterial rRNA remaining after depletion?
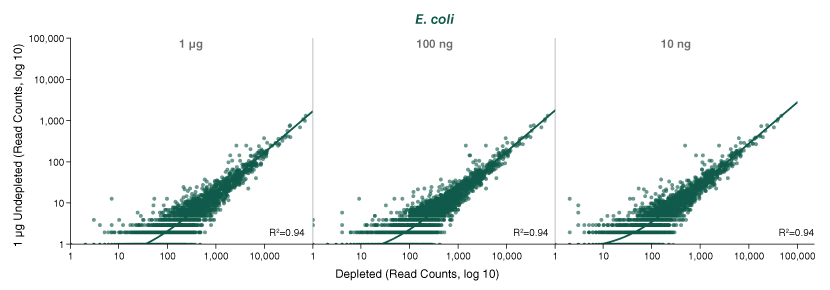
- How can I determine if the rRNA depletion was efficient?
- Can I use this product with degraded RNA or fragmented RNA?
- What is the total RNA input I should use?
- Why must the RNA be free of DNA?
- Which microbiome community was used in the depletion experiments described, and which references were used to assess the performance of the NEBNext® rRNA Depletion Kit (Bacteria) with this sample?
- Which species are compatible with the NEBNext® rRNA Depletion Kit (Bacteria)?
- Which species have been tested with the NEBNext rRNA Depletion Kit (Bacteria)?
- Can the enriched RNA resulting from the NEBNext depletion kit be used in long read sequencing applications?
Troubleshooting
Citations & Technical Literature
Citations
Additional Citations
Quality, Safety & Legal
Quality Assurance Statement
Quality Control tests are performed on each new lot of NEB product to meet the specifications designated for it. Specifications and individual lot data from the tests that are performed for this particular product can be found and downloaded on the Product Specification Sheet, Certificate of Analysis, data card or product manual. Further information regarding NEB product quality can be found here.Specifications
The Specification sheet is a document that includes the storage temperature, shelf life and the specifications designated for the product. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]Safety DataSheets
The following is a list of Safety Data Sheet (SDS) that apply to this product to help you use it safely.NEBNext® DNase I
NEBNext® Thermostable RNase H
NEBNext® Bacterial rRNA Depletion Solution
RNase H Reaction Buffer
DNase I Reaction Buffer
Nuclease-free Water
NEBNext Probe Hybridization Buffer
NEBNext® RNA Sample Purification Beads
Legal and Disclaimers
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email [email protected].This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
New England Biolabs (NEB) is committed to practicing ethical science – we believe it is our job as researchers to ask the important questions that when answered will help preserve our quality of life and the world that we live in. However, this research should always be done in safe and ethical manner. Learn more.
Other Products You May Be Interested In
-

NEBNext® Ultra™ II Directional RNA Library Prep Kit for Illumina®
-

NEBNext® Ultra™ II Directional RNA Library Prep with Sample Purification Beads
-

NEBNext® rRNA Depletion Kit (Human/Mouse/Rat)
-

NEBNext® Globin & rRNA Depletion Kit (Human/Mouse/Rat)
-

NEBNext® Globin & rRNA Depletion Kit (Human/Mouse/Rat) with RNA Sample Purification Beads
-

NEBNext® Poly(A) mRNA Magnetic Isolation Module
The supporting documents available for this product can be downloaded below.




