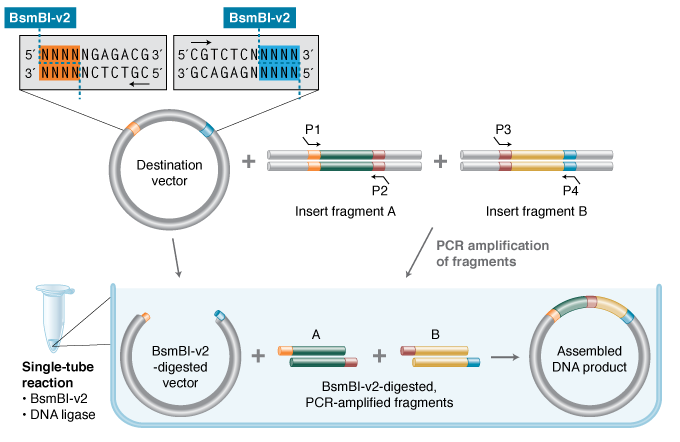
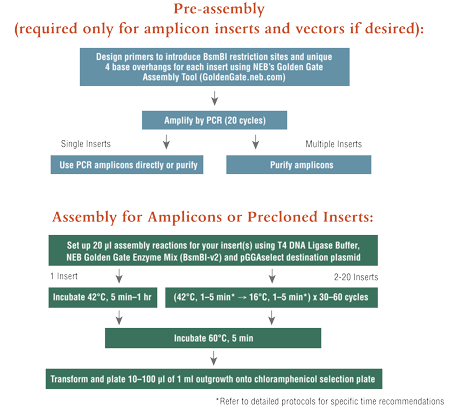
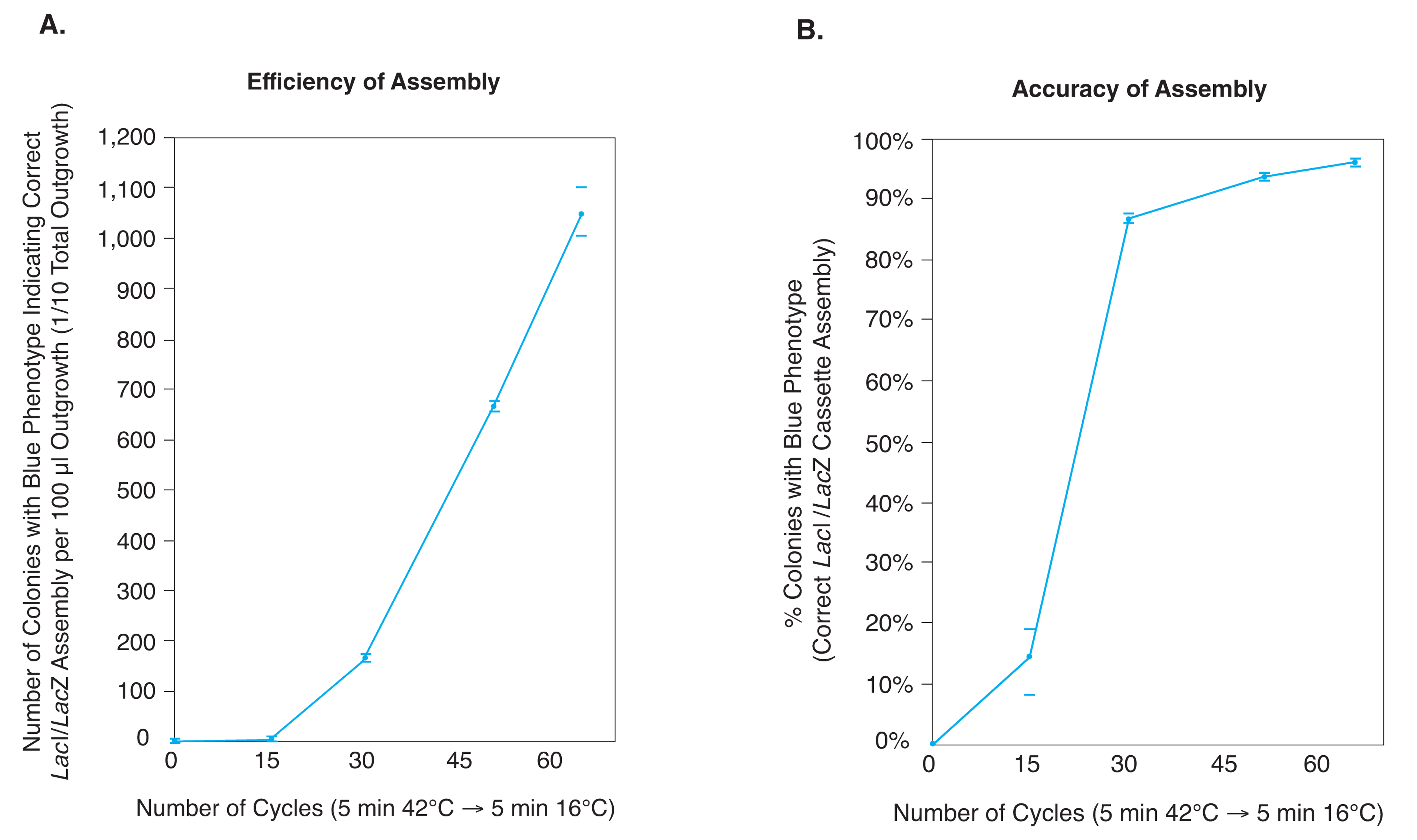
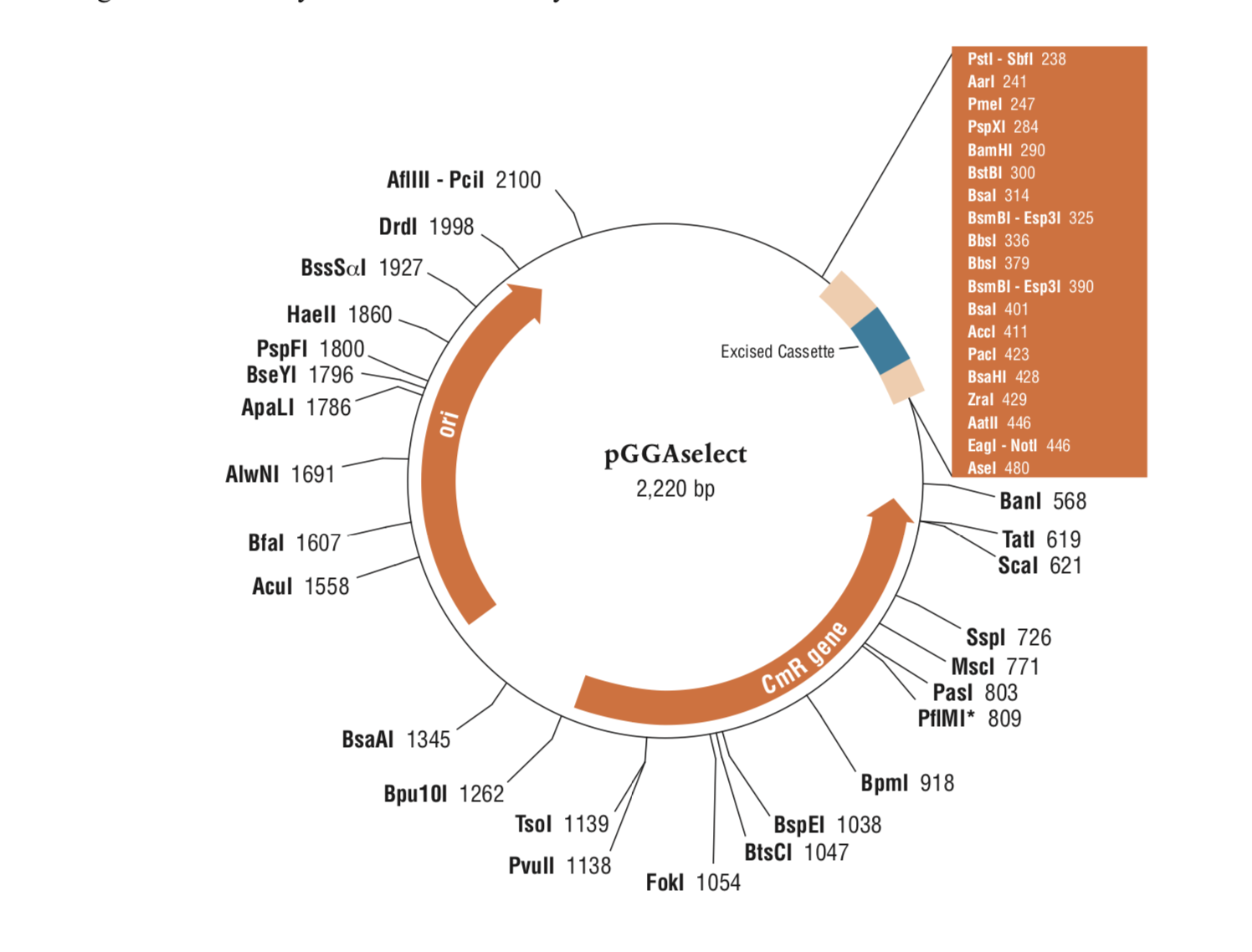
NEBridge® Golden Gate Assembly Kit (BsmBI-v2)
Product information| Code | Name | Size | Quantity | Price | |
|---|---|---|---|---|---|
E1602S |
NEB Golden Gate Assembly Kit (BsmBI-v2) |
20 rxns | - | Unavailable in your region | |
E1602L |
NEB Golden Gate Assembly Kit (BsmBI-v2) |
100 rxns | - | Unavailable in your region |