Monarch® RNA Cleanup Kit (10 μg)
Product information| Code | Name | Size | Quantity | Price | |
|---|---|---|---|---|---|
T2030S |
Monarch RNA Cleanup Kit (10 µg) |
10 preps | - | Unavailable in your region | |
T2030L |
Monarch RNA Cleanup Kit (10 µg) |
100 preps | - | Unavailable in your region |
Monarch® RNA Cleanup Kit (10 μg)
Effective March 25, 2024, component/product name changed to Monarch® Buffer BX. Effective March 25, 2024, component/product name changed to Monarch® Buffer WX.
Product Introduction
The Monarch RNA Cleanup Kit (10 µg) enables fast and simple purification and concentration of up to 10 µg of RNA from enzymatic reactions.
- Ideal for cleanup and concentration of RNA after enzymatic treatments including DNase I, Proteinase K, labeling, capping or in vitro transcription (IVT)
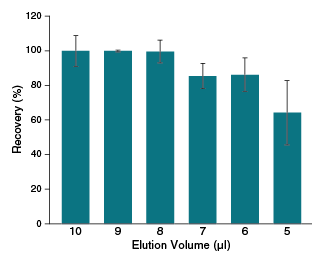
- Elute in ≥ 6 µl for concentrated RNA
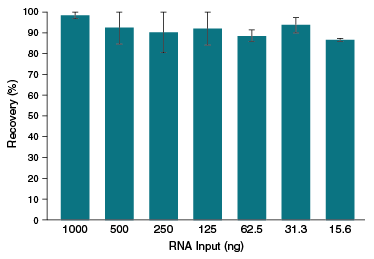
- 70-100% RNA recovery, even with inputs < 20 ng
- Efficiently purify RNA ≥ 25 nt (a simple modification enables purification of RNA ≥ 15 nt)
- Can be used to purify RNA from the aqueous phase following TRIzol® or similar extractions
- Simplified workflow with a single wash buffer
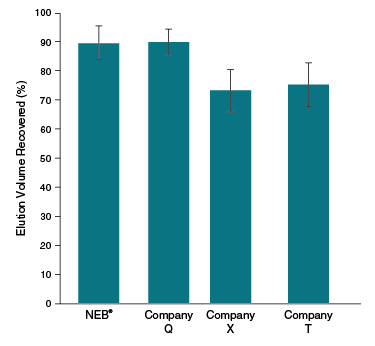
- Unique column design prevents buffer carryover and elution of silica particulates
- Columns and buffers are available separately
- Purified RNA is ready for use in a wide variety of downstream applications
Check out our Technical Note containing comprehensive insights into measuring and analyzing nucleic acids.
| Catalog # | Size | Concentration |
|---|---|---|
| T2030S | 10.0 preps | |
| T2030L | 100.0 preps |
- Product Information
- Protocols, Manuals & Usage
- FAQs & Troubleshooting
- Citations & Technical Literature
- Quality, Safety & Legal
- Other Products You May Be Interested In
Product Information
Description
The Monarch RNA Cleanup Kit (10 µg) rapidly and reliably purifies up to 10 μg of concentrated, high-quality RNA (> 25 nt) from enzymatic reactions including labeling, capping, in vitro transcription (IVT) and DNase I treatment. This kit utilizes a bind/wash/elute workflow with minimal incubation and spin times. Our unique column design ensures zero buffer retention and no carryover of contaminants, enabling elution of sample in volumes as low as 6 μl. Eluted RNA is ready for use in a variety of downstream applications, including RT-PCR, RNA Library Prep for NGS and RNA labelling. The protocol can also be modified to enable the purification of smaller RNA fragments (≥ 15 nts).
Designed with sustainability in mind, Monarch kits use significantly less plastic and responsibly-sourced, recyclable packaging.
Monarch RNA Cleanup kits are also available for 50 µg (NEB #T2040) and 500 µg (NEB #T2050) binding capacities. Columns and buffers are also available separately for convenience.
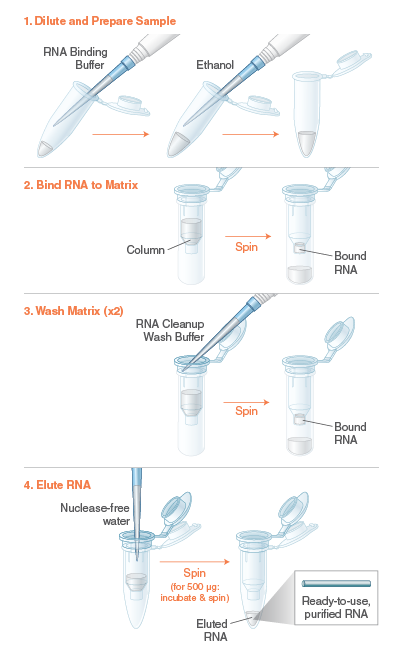
Specifications and Applications:
| SPECIFICATIONS | |
|---|---|
| RNA Sample Type | Cleanup and concentration of RNA from enzymatic reactions (labeling, capping, in vitro transcription reactions, DNase I treatment) |
| Binding Capacity | 10 μg |
| RNA Size Range | ≥ 25 nt ( ≥ 15 nt with modified protocol) |
| Typical Recovery | 70–100% |
| Elution Volume | 6–20 µl |
| Purity | A260/280 > 1.8 and A260/230 > 1.8 |
| Protocol Time | 5 minutes of spin and incubation time |
| Compatible Downstream Applications | RT-PCR, Small RNA library prep for NGS, RNA Library Prep for NGS |
| APPLICATIONS | |
|---|---|
| RNA Cleanup and Concentration (including from the TRIzol aqueous phase) | RNA purified by other methods can be further purified |
| Enzymatic Reaction Cleanup | Enzymes such as RNA polymerases, DNase I, Proteinase K and phosphatases are removed allowing efficient desalting |
| In vitro Transcription Cleanup | Enzymes and excess NTPs are removed to yield highly pure synthesized RNA |
| RNA Gel Extraction | Purification of RNA from agarose gels |
| RNA Fractionation | Fractionation of RNA into small and large RNA pools |
- This product is related to the following categories:
- RNA Cleanup Products,
- RNA Extraction and Purification,
- Nucleic Acid Purification Products,
Kit Components
Kit Components
The following reagents are supplied with this product:
| NEB # | Component Name | Component # | Stored at (°C) | Amount | Concentration | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
Properties & Usage
Protocols, Manuals & Usage
Protocols
- Monarch® RNA Cleanup Kit Protocol
- Purification of RNA from the Aqueous Phase Following TRIzol®/Chloroform Extraction using the Monarch® RNA Cleanup Kits
- Separation of Large and Small RNA into Fractions using the Monarch® RNA Cleanup Kits
- Extraction of RNA from Agarose Gels using the Monarch® RNA Cleanup Kits
- RNA Extraction from Cells Using the Monarch RNA Cleanup Kits
- RNA Extraction from Buccal/Nasopharyngeal Swabs Using the Monarch RNA Cleanup Kits
Manuals
Usage & Guidelines
Application Notes
FAQs & Troubleshooting
FAQs
- What is the composition of each buffer provided with the Monarch RNA Cleanup Kits?
- What is the maximum binding capacity of the Monarch RNA Cleanup Column provided with the Monarch RNA Cleanup Kit?
- What is the smallest volume of nuclease-free water that can be used for elution with the Monarch RNA Cleanup Columns?
- Can I get better recovery with the Monarch RNA Cleanup Kits if I do a second elution with my eluent from the first elution?
- What factors affect my (A260/A230) when using the Monarch RNA Cleanup Kits?
- What size RNA can be purified with the Monarch RNA Cleanup Kit?
- Can I use the Monarch RNA Cleanup Kit to cleanup up my DNase I-treated RNA?
- Do you have a protocol for separating small and large RNAs into separate fractions?
- Can I use the Monarch RNA Cleanup Kits to purify RNA from agarose gels?
- Can I use the Monarch RNA Cleanup Kits to cleanup RNA after a TRIzol®/chloroform extraction?
- Are the Monarch RNA Cleanup Kits compatible with Luna RT-qPCR reagents?
- Are the Monarch RNA Cleanup Kits compatible with NEBNext reagents for RNA library prep?
- How can I assess RNA integrity and purity?
- Are the columns in the Monarch RNA Cleanup Kits the same as those in the Monarch Total RNA Miniprep Kit (NEB #T2010)?
- Can I purchase Monarch® buffers and columns separately?
- Can I do an on-column DNase I treatment with the Monarch RNA Cleanup Columns?
- Can the Monarch RNA Cleanup Kits (NEB #T2030, #T2040, #T2050) also be used to purify DNA?
- Can the Monarch RNA Cleanup Kits be used for RNA extraction?
- I noticed the buffer names have changed in the RNA Cleanup Kits. Are these buffers the same formulation as before?
Troubleshooting
| Problem | Cause | Solution |
|---|---|---|
| Low RNA Yield | Reagents added incorrectly | Check protocol to ensure correct buffer reconstitution, order of addition of buffers and ethanol, and proper handling of column flow-through and eluents. |
| Insufficient mixing of reagents | Ensure the ethanol is thoroughly mixed with RNA sample and RNA Cleanup Binding Buffer before applying the sample to the RNA Cleanup Column. | |
| Incomplete elution during prep | Ensure the nuclease-free water used for elution is delivered directly to the center of the column so that the matrix is completely saturated. Larger elution volumes, multiple elutions, and longer incubation times can increase yield of RNA, but will dilute the sample and may increase processing times. For typical RNA samples, the recommended elution volumes and incubation times should be sufficient. | |
| High degree of RNA secondary structure | Binding and elution of smaller RNAs (< 45 nt) can be affected by secondary structure of the RNA molecules. If poor yield of a small RNA is observed, we recommend diluting your sample with 2 volumes of ethanol instead of one volume in Step 2 of the protocol. | |
| Purified RNA is Degraded | RNase contamination | In order to avoid RNase contamination during RNA cleanup, make sure to work on a clean lab bench, wear gloves and use disposable RNase-free pipet tips and microfuge tubes (not provided). Keep all kit components tightly sealed when not in use. |
| Improper storage of RNA | Purified RNA should be used immediately in downstream applications or stored at -70°C. | |
| Low A260/230 Ratios | Residual guanidine salt carry-over | Ensure wash steps are carried out prior to eluting sample. Use care to ensure the tip of the column does not contact the flow-through. If unsure, repeat centrifugation. When reusing collection tubes, blot the rim of the tube on a Kimwipe prior to reattachment to the column to remove any residual wash buffer. |
| Low Performance of RNA in Downstream Steps | Salt and/or ethanol carry-over | Ethanol and salt remaining after the washes may inhibit downstream applications. Use care to ensure that the tip of the column does not come into contact with the flow-through. If in doubt, re-centrifuge for 1 minute to ensure traces of salt and ethanol are not carried over in the eluted RNA. |
| DNA contamination | DNA removal may be necessary for certain applications. Incubate RNA sample with DNase I (NEB #M0303) and cleanup RNA using the Monarch RNA Cleanup Protocol. |
Citations & Technical Literature
Citations
Additional Citations
Quality, Safety & Legal
Quality Assurance Statement
Quality Control tests are performed on each new lot of NEB product to meet the specifications designated for it. Specifications and individual lot data from the tests that are performed for this particular product can be found and downloaded on the Product Specification Sheet, Certificate of Analysis, data card or product manual. Further information regarding NEB product quality can be found here.Specification Change Notifications
Effective March 25, 2024, component/product name changed to Monarch® Buffer BX. Product specifications have been updated.
Effective March 25, 2024, component/product name changed to Monarch® Buffer WX. Product specifications have been updated.Specifications
The Specification sheet is a document that includes the storage temperature, shelf life and the specifications designated for the product. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]Certificate Of Analysis
The Certificate of Analysis (COA) is a signed document that includes the storage temperature, expiration date and quality controls for an individual lot. The following file naming structure is used to name these document files: [Product Number]_[Size]_[Version]_[Lot Number]- T2030L_v1_0011806
- T2030S_v1_0011806
- T2030S_v1_10023551
- T2030L_v1_10041690
- T2030S_v1_10041689
- T2030L_v1_10023550
- T2030L_v1_10068598
- T2030S_v1_10068599
- T2030L_v2_10099678
- T2030L_v1_10059911
- T2030S_v1_10059912
- T2030S_v2_10099682
- T2030L_v3_10126366
- T2030L_v3_10143529
- T2030S_v3_10126367
- T2030L_v3_10144609
- T2030S_v3_10144710
- T2030L_v3_10144718
- T2030S_v3_10144721
- T2030L_v3_10163944
- T2030S_v3_10163947
- T2030L_v3_10197170
- T2030S_v3_10197177
Safety DataSheets
The following is a list of Safety Data Sheet (SDS) that apply to this product to help you use it safely.Monarch® Buffer BX
Monarch® Buffer WX
Nuclease-free Water
Legal and Disclaimers
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email [email protected].This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.
New England Biolabs (NEB) is committed to practicing ethical science – we believe it is our job as researchers to ask the important questions that when answered will help preserve our quality of life and the world that we live in. However, this research should always be done in safe and ethical manner. Learn more.
Other Products You May Be Interested In
-

LunaScript® RT SuperMix Kit
-
DNase I (RNase-free)
-

Monarch® RNA Cleanup Columns (10 μg)
-
Monarch® RNA Cleanup Kit (50 μg)
-
Monarch® RNA Cleanup Kit (500 μg)
-
Monarch® Total RNA Miniprep Kit
-

Monarch® RNA Cleanup Columns (50 μg)
-

Monarch® RNA Cleanup Columns (500 μg)
-
Monarch® Collection Tubes II
-
Monarch® Buffer BX
-

Monarch® Buffer WX
-
Nuclease-free Water
The supporting documents available for this product can be downloaded below.