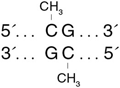
CpG Methyltransferase (M.SssI)
Product information| Code | Name | Size | Quantity | Price | |
|---|---|---|---|---|---|
M0226S |
CpG Methyltransferase (M.SssI) |
100 units ( 4000 units/ml ) | - | Unavailable in your region | |
M0226L |
CpG Methyltransferase (M.SssI) |
500 units ( 4000 units/ml ) | - | Unavailable in your region | |
M0226M |
CpG Methyltransferase (M.SssI) |
500 units ( 20000 units/ml ) | - | Unavailable in your region |